In today's society, the incidence of cardiovascular and cerebrovascular diseases continues to rise. Against this backdrop, balloon catheters, as key medical devices for the treatment of cardiovascular and cerebrovascular diseases, are increasingly applied and rapidly developing. This article will delve into the related content of medical balloons, including their molding technology, material requirements, composition, clinical applications, and prospects for future development.

SmartBalloon OEM/ODM Solutions – Vertebral Expansion Balloon
I. The Development of Cardiovascular and Cerebrovascular Diseases and Interventional Treatment
With the improvement of living standards and the acceleration of life pace, cardiovascular and cerebrovascular diseases have become more common. In the 1980s, Swedish doctors successfully carried out percutaneous transluminal coronary angioplasty, marking the birth of interventional medicine. Vascular interventional treatment uses interventional catheters to reach the lesion through the vascular cavity, achieving minimally invasive treatment. In the past three decades, it has developed rapidly due to its many advantages. Its application range is wide, covering the entire body, such as intracranial nerves, carotid arteries, renal arteries, veins, etc. Balloon catheters, as the main devices for endovascular interventional treatment, are the most commonly used type of catheter in clinical practice.
II. Materials and Requirements for Medical Catheter Molding
Medical catheter materials are abundant, mostly thermoplastic polymers, with dozens of organic molecular materials used for different purposes. Common materials include silicone rubber, polyurethane (PU), polytetrafluoroethylene (PTFE), polyethylene (PE), polypropylene (PP), polyvinyl fluoride (PVF), polymethyl methacrylate (PMMA), polyethylene terephthalate (PET), nylon (PA), polycarbonate (PC), etc. Medical catheters have the following basic requirements:
1. Thermal Stability: Able to be sterilized in an autoclave using radiation or other methods, with good thermal stability.
2. Chemical Stability: It is anti-oxidation and corrosion-resistant, chemically stable in contact with body fluids, and chemically inert.
3. Biocompatibility: Good tissue and blood compatibility, anti-thrombotic, non-coagulating.
4. Safety: Non-carcinogenic and does not cause allergic reactions.
5. Mechanical Properties: Excellent mechanical properties and good functionality, not losing tensile strength and modulus of elasticity when implanted in the body for a long time.
6. Processability: Excellent processability.
III. Evolution of Medical Balloon Materials
Medical balloons are usually made of polymer materials. Initially, soft polyvinyl chloride was used, with a number-average molecular weight of about 36,000 – 93,000 and a degree of polymerization of 590 – 1500. It has good chemical stability, resistance to organic solvents and chemicals, stable to acids, bases, and salts at room temperature, and excellent mechanical, mechanical, and electrical properties. However, it has poor light and heat resistance, a softening point of 80°C, a high glass transition temperature, a melting point close to the decomposition temperature, high hardness, and difficulty in processing, resulting in thick balloon walls but poor pressure resistance. Recent studies have found that polyvinyl chloride has poor biocompatibility, and monomers have carcinogenic effects.
Since the early 1980s, cross-linked polyethylene and polyethylene terephthalate (PET) have been used to make balloons, significantly improving performance, with more than 90% of balloons made from these materials at that time. Polyethylene has good chemical stability, is stable to acids, bases, and salts at room temperature, has high insulation, strong corrosion resistance, and good biocompatibility; PET is an aromatic ring-containing linear polyester with good rigidity, strong creep resistance, high dimensional stability, and low moisture absorption. The higher the viscosity of PET, the lower the crystallinity, and the burst pressure can reach 27atm. Balloons made from cross-linked polyethylene have good shape retention, but these materials are hard and have practical application shortcomings.
In the late 1980s and early 1990s, nylon and thermoplastic polyurethane materials were gradually used for balloons. Thermoplastic polyurethane is a linear polymer containing hard segments and elastic soft segments, with good processability, biocompatibility, and wear resistance, but poor resistance to hydrolysis and cannot be steam sterilized. Nylon has high mechanical strength, toughness, high tensile and compressive strength, excellent fatigue resistance, smooth surface, low friction coefficient, corrosion resistance, and strong aging resistance, but high water absorption and poor light resistance, and the production of nylon balloons in China is relatively large. New balloon materials are mostly copolymers or compatible polymer mixtures of rigid chain segments and flexible chain segments. Alloys of polytetrafluoroethylene with nylon, polyurethane, and other materials are the preferred materials for producing high-grade interventional catheters, with excellent performance but difficult processing. With the development of science and technology, more high-performance polymer materials will be used in balloon production, promoting medical progress.

IV. Domestic and International Research Progress on Medical Balloons
Abroad, in the United States Patent Description, general balloon molding methods and improvements were introduced, placing the mold in a heated conductive medium, placing the thermoplastic material tube blank in the mold, inflating the balloon while stretching the tube blank to form the balloon. Murthy V.S imhambhat la. et al. introduced the over-the-wire production process, first continuously or semi-continuously expanding the extruded tube blank into an expansion tube, and then further expanding it into a balloon in the balloon cavity. Jarakani of Orbus Medical Technology Co., Ltd. developed a balloon catheter suitable for percutaneous transluminal coronary angioplasty, including a catheter body and an expandable balloon, with a hard proximal end, a soft distal end, and a soft element connecting the middle. Wang Zhaohua and Du Hui et al. designed a contrast balloon dilator, sealing one end of the catheter, covering the inner and outer balloons and tightening, placing a contrast agent solution between the two balloons, and matching an inflatable balloon and a pressure gauge. After the balloon is inflated, it can be clearly visualized under X-ray, suitable for the expansion of stenosis in the esophagus, large vessels, and heart valve areas. Ma Ruikun proposed a method for making medical polyethylene balloon catheters, using cobalt source radiation to cross-link polyethylene, placing the tube in the mold, inflating and stretching while heating, and blowing and stretching at the right temperature and pressure, cooling and setting, resulting in excellent balloon performance, which can be used to treat diseases such as pulmonary valve stenosis.
Domestically, although there are patents in balloon production, the start is relatively late, and there is a gap with foreign countries. The production of medical balloon catheters requires high environmental, material, and technical requirements, and is constrained by production equipment and supporting processes. A large number of balloons used in China rely on imports.
V. Clinical Applications of Medical Balloons
Balloon catheters are widely used in the treatment of vascular diseases, commonly used to treat arterial stenosis. Doctors guide the catheter with an expansion balloon into the blood vessel under fluoroscopy, position it at the lesion, and then pressurize to expand the balloon for treatment. This method is minimally invasive, easy to operate, and has few complications, replacing surgical operations with internal intervention, reducing patient pain.
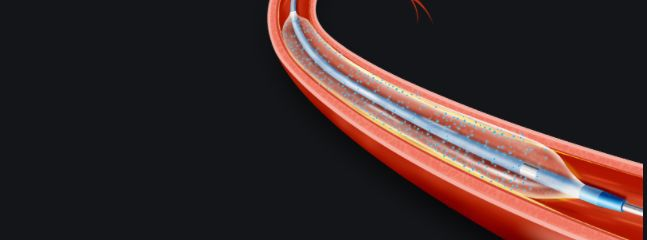
Medical balloons are most widely used in percutaneous transluminal coronary angioplasty (PTCA). After the invention of drug stents, the use of balloon catheters has been expanded, in addition to treating vascular stenosis, expansion, and shaping, they are also used for stent delivery, expansion, pre-expansion before placement, and precise shaping after placement, widely applied in coronary arteries, intracranial nerves, renal arteries, femoral arteries, bile ducts, and gastrointestinal systems. In orthopedics, for patients with osteoporotic vertebral compression fractures, medical balloons have significant effects, can reduce pain, and improve treatment effects. The operation method is to place an inflatable bone balloon into the vertebra through the skin and pedicle, inflate the balloon to reposition the fractured vertebra, create space after deflating and exiting the balloon, and then inject bone cement at low pressure.
In addition, medical balloons are also used in new fields such as laser catheters and photoactivation treatment, and drug delivery catheters. Balloons used for laser catheters and photoactivation treatment are made of transparent, low light absorption materials, with a laser installed inside, and the cone and tube segments are coated with non-transparent materials, only the working segment is transparent. When in use, the balloon is positioned and expanded at the lesion site, and the laser treatment is activated at the lesion site, which can effectively control the treatment area. The balloon of the drug delivery catheter has micropores, and when the balloon is expanded at the lesion site, the micropores allow drugs to pass through, treating local lesions, and after the balloon deflates, the drug can return to the balloon, reducing the amount of medication and avoiding damage to normal tissue.
VI. SmartBalloon Medical Technology Co., Ltd.
SmartBalloon Medical is a technology company focusing on providing customers with one-stop OEM/ODM services for medical balloon sacs, minimally invasive interventional catheter technology product solutions, contract manufacturing, and customized production. The founding team members have more than 15 years of experience in medical balloon catheter technology, and the team is committed to providing more professional technology, better quality levels, and more economical costs for medical balloon catheter enterprises through more professional technology, better quality levels, and more economical costs. The company is located in the Jiaxing Guide Industrial Park, which is part of the Yangtze River Delta Integration Demonstration Zone.
In terms of technology research and development, SmartBalloon Technology has invested heavily, established an advanced R&D center, equipped with world-class experimental equipment and testing instruments, and is constantly exploring and innovating to improve product performance and quality. SmartBalloon has achieved many breakthroughs in the research and development of medical balloons, able to precisely control the molding process of balloons to ensure that the size accuracy, pressure resistance, and flexibility of the balloons achieve the best balance.

In the production process, SmartBalloon Technology has a clean workshop that meets international standards and strictly follows the quality management system. Every link from raw material procurement to finished product output is subject to strict quality testing to ensure the safety and reliability of the products. Our production process is advanced, and we can efficiently produce high-quality medical balloons to meet the requirements of Party A.

In terms of product quality, SmartBalloon Technology's medical balloons have significant advantages for corporate users. We carefully select high-quality polymer materials and combine advanced processing technology to ensure that the balloons have good biocompatibility, excellent pressure resistance, and outstanding flexibility.
In clinical application scenarios, our products have shown very high stability and reliability, helping corporate customers gain an advantage in market competition, and also bringing better treatment effects to patients.
VII. Prospects for the Development of Medical Balloons
With the in-depth study of polymer materials and the improvement of balloon manufacturing technology, the application prospects of medical balloons are broad. The future development focus will be on tumor interventional treatment, cardiac interventional technology, neuro-interventional technology, non-vascular endovascular interventional technology, portal hypertension vascular endovascular interventional treatment, non-tumor lesion and peripheral vascular lesion endovascular interventional treatment technology, emergency bleeding arterial embolization treatment, and interventional treatment at the cellular and molecular (gene) level. The development direction of medical balloons is to be more excellent and refined in performance, including thinner walls, better pressure resistance, and better flexibility, while finding a balance among the three, and because the uses and requirements of balloons in different fields are different, the production will be more refined and specialized.
References:
[1] Chen Xingrong. Selective Vascular Angiography [M]. Shanghai: Shanghai Science and Technology Press, 1990: 1-38.
[2] Qian Huohong, Xu Qin, Qiu Qun, et al. Clinical Application of Central Venous Catheter Replacement [J]. Chinese Journal of Nursing, 2004, 39: 143-144.
[3] Bai Mu, Zhou Jie. The Application of Plastics in the Medical and Health Field [J]. Engineering Plastics Application, 2003, 31: 47-49.
[4] Mark A. Saab. Applications of High-Polymer Balloons in the Medical Device Industry [J]. Device & Diagnostic Industry, 2000.
[5] Chen Jun, Chen Jianling, et al. Research Progress on PET Crystallization Behavior [J]. Polymer Bulletin, 2005, 1: 20-24.
[6] Yang Hai, Shi Xiufeng. Proceedings of the First National Interventional Medical Engineering Academic Conference [C]. 2007: 97-103.
[7] L. Wang, P. Miller, D. Horn, et al. PROCESS IMPROVEMENTS FOR PREPARING CATHETER BALLOONS [P]. Google Patents, 1998.
[8] M. Simhambhatla, Method of making a catheter balloon [P]. Google Patents, 2001.
[9] M. Jilakani. Balloon Catheter [P]. Chinese Patent: 03815912.0, 2005-09-07.
[10] Wang Zhaohua, Du Hui. Contrast Balloon Dilation Device [P]. Chinese Patent: 94235559.8, 1995-09-27.
[11] Ma Ruikun. Production of Medical Polyethylene Expansion Balloon [P]. Chinese Patent: 89107579.8, 1990-03-28.
[12] Guo Lihua, Wang Wan, Wang Binxiu. Design of Expansion Die for Medical Interventional Catheters [J]. Plastics, 2005, 34(4): 72-74.
[13] Yang Huilin. Balloon Kyphoplasty for the Treatment of Osteoporotic Vertebral Compression Fracture [J]. Journal of Soochow University: Medical Edition, 2002, 22(4): 406-409.
[14] James J. Glazer, MD, Alice Jet al. Laser balloon angioplasty combined with local intracoronary heparin therapy: Immediate and short-term follow-up results [J]. American Heart Journal, 1997, 134.
www.smartballoon.net
SmartBalloon Medical Technology